This article from the The Lancet Respiratory Medicine magazine is the ventilation study referred to in Webinar 2
Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission
Globally, health-care authorities are searching for effective measures to prevent community transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although data on factors related to this transmission are scarce, the spread of SARS-CoV-2 is thought to mostly be via the transmission of respiratory droplets coming from infected individuals.1 Small droplets, from submicron to approximately 10 μm diameter, produced during speech and coughing, have been shown to contain viral particles,2 which can remain viable and infectious in aerosols for 3 h.3 The droplets can be transmitted either directly by entering the airway through the air (aerosols),4 or indirectly by contact transfer via contaminated hands. The mode of transmission could affect whether an infection starts in the upper or lower respiratory tract, which is thought to affect the severity of the disease progression.5 Notably, the dose–response relationship of SARS-CoV-2 infection is still unclear, especially with respect to aerosol transmission of the virus. However, aerosols containing a small concentration of virus in poorly ventilated spaces, combined with low humidity and high temperature,6 might result in an infectious dose over time.
To better understand the spreading of respiratory droplets and possible preventive measures, we analysed droplet production due to coughs and speech by measuring the droplet size distribution, travel distance and velocity, and the airborne time in relation to the level of air ventilation.
We did a laser diffraction measurement using a spray droplet measurement system (Malvern Spraytec, Malvern, UK) to determine the size distribution of respiratory droplets in a single cough and during speech. In a cough from a healthy volunteer, we found two distinct types of drops, large droplets (100–1000 μm in diameter) and small droplets (1–10 μm), with the small droplets being much more prevalent (appendix p 1). During speech, only the small droplets were found (appendix p 1). Although large droplets have been specifically related to coughs,4 here we observe that both sizes of droplet are produced by coughing.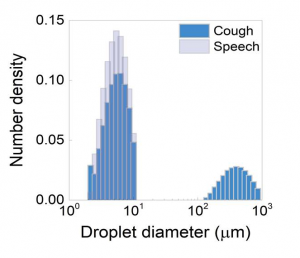
appendix p 1
Next, we used a SprayScan (Spraying Systems, Glendale Heights, IL, USA) laser sheet to track droplets by filming the scattering of laser light by droplets from a cough7 to determine the speed of the droplets and their trajectory. Large droplets were observed to fall onto the ground rapidly (appendix p 2). We found that although the speed of the drops ranged 2–7 m/s at the start of the cough, the visible large drops (typically 500 μm in diameter) do not travel far before their trajectory bends down due to gravity to rapidly fall onto the ground within 1 s. This observation can be explained by balancing the forces of gravity (F=mg; where F is force, m is mass, and g is acceleration) and air drag (F=6πηRU, in which η is the air viscosity, R is the radius of the droplet, and U is the falling velocity), from which it also follows that the small droplets of typical radius of 5 μm will take 9 min to reach the ground when produced at a height of 160 cm (ie, average speaking or coughing height). These small droplets are of specific interest because they have been associated with aerosol transmission of the SARS-CoV-2.8 We also investigated droplets coming from the nasal cavity, and found that with normal breathing no droplets are detected above the background noise level (2·3 [SD 1·5] droplets, and 2·6 [1·7] droplets for nasal breathing). From a sneeze, we found mostly very large drops, originating from both the buccal and nasal cavities, that are not persistent.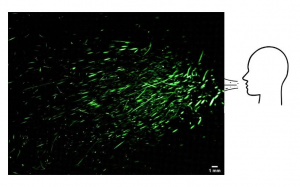
appendix p 2
The same laser sheet was used to investigate how long small droplets from a cough will float through the air. We used a specially designed spray nozzle from Medspray (Enschede, The Netherlands) to disperse a controlled quantity of small droplets into the air, reproducing the effect of coughing. The droplets have an average diameter of 5 μm and are dispersed homogeneously by the spray nozzle. We analysed the number of droplets passing through the stationary laser sheet suspended in the centre of the experimental chamber using an algorithm that detects the illuminations caused by the droplets. We repeated this experiment in three rooms with different levels of ventilation: no ventilation, mechanical ventilation only, and mechanical ventilation supported by the opening of an entrance door and a small window (appendix p 3). In the best ventilated room, after 30 s the number of droplets had halved, whereas with no ventilation this took about 5 min, in agreement with the air drag calculation that shows that 5 μm drops from the average cough or speech height take 9 min to reach the ground. In a poorly ventilated room, the number of droplets was halved in 1·4 min.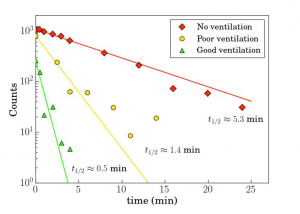
appendix p 3
Although we only studied healthy volunteers and did not study patients with COVID-19 or virus-laden aerosol droplets directly, our data on droplet size distribution and persistence does have implications on requirements to use face masks to prevent virus transmission. Transmission by aerosols of the small droplets studied here can only be prevented by use of high-performance face masks; a conventional surgical mask only stops 30% of the small aerosol droplets studied here for inhaled breath;9 for exhaled breath the efficacy is much better.10
Additionally, the long airborne time of aerosols we found here affects the reliability of temporal and spatial contact data between individuals as monitored by proximity tracing via smartphone apps. These findings need to be considered in the development and implementation of these apps.
This study shows that better ventilation of spaces substantially reduces the airborne time of respiratory droplets. This finding is relevant because typically poorly ventilated and populated spaces, like public transport and nursing homes, have been reported as sites of viral transmission despite preventive physical distancing. The persistence of small respiratory droplets in such poorly ventilated spaces could contribute to the spread of SARS-CoV-2. Our findings confirm that improving ventilation of public spaces will dilute and clear out potentially infectious aerosols. To suppress the spread of SARS-CoV-2 we believe health-care authorities should consider the recommendation to avoid poorly ventilated public spaces as much as possible. The implications are also important for hospital settings where aerosolisation by coughing and medical treatments and close contact with COVID-19 patients is very common.


